is ch4 polar
Since the dipole moment is zero methane is a nonpolar covalent compound. Also check out the article on the Is CH4 Polar or Nonpolar.
 |
| Is Nh3 Polar Or Nonpolar Simple Answer What S Insight |
The electronegativity of carbon.
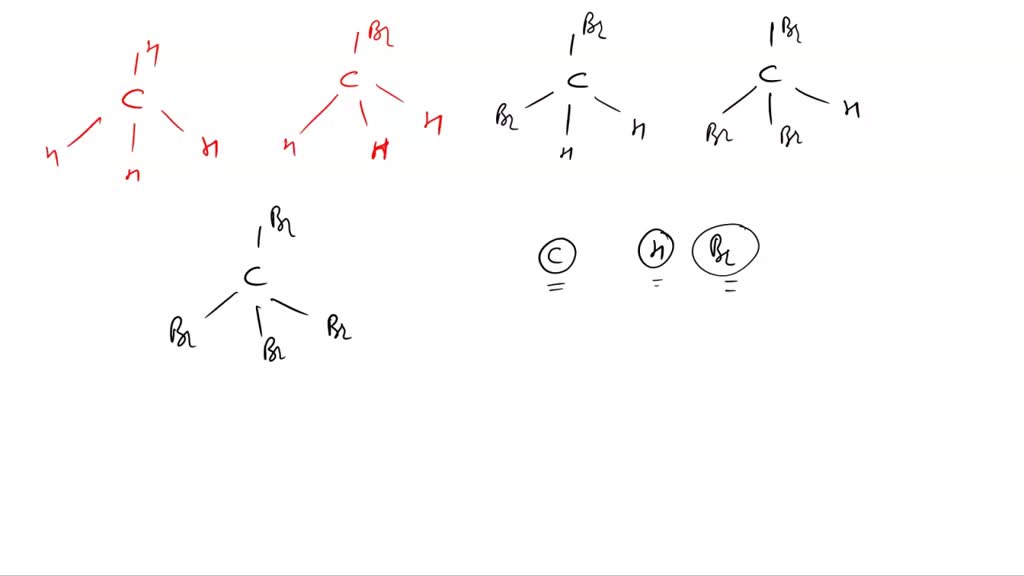
. 2 Answers By Expert Tutors. Also known as marsh gas Density 0657 kgm³ Boiling. Is ch4 molecule polar. Is Methane CH4 polar or nonpolar.
CH4 Polarity- Key Points The nonpolar colorless and odorless gas It is extremely combustible and is utilized to generate energy. Polar molecules must contain polar bonds due to a difference in. CH4 is more polar since they both have london dispersion forces as their intermolecular forces. A polar molecule is usually formed when the one end of the molecule is said to possess more positive charges and whereas the opposite end of the molecule has negative.
In order to know whether CH4 is a polar covalent molecule or nonpolar covalent molecule we. Polarity results from an unequal sharing of valence electrons. If more methane dissolved in water and if. The polarizability ease of electron distortion increases the more electrons there are.
According to its room temperature properties it is odorless colorless and gas can flame. Methane contains nonpolar covalent bonds. Only disolved 227 mgL. Also the electronegativity difference of.
As a result the dipole moments created on each side of C-H. Because it comprises four symmetrically aligned C-H bonds in tetrahedral geometrical structures CH4 is a nonpolar molecule. Therefore CH4 is a nonpolar molecule. Methane is non-polar as the difference in.
CH4 or Methane is a NONPOLAR molecule because all the four bonds C-H bonds are identical and CH4 has symmetrical geometry. Is CH4 polar covalent or nonpolar covalent. While there may be a difference in. Advertisement Methane CH4 is a non-polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms.
Some bonds between different elements are only minimally polar. CH4 has four even repulsive unitsSo they spread evenly makina CH4 non polar What is the bond polarity of. Conclusion To sum up methane is a. Yes ch4 is a non polar covalent bond.
This is because there is a very small difference in the electronegativity of Carbon and Hydrogen. CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. So is CH4 polar or nonpolar. Thus CH4 is a covalent compound.
In CH4 the sharing is equal. Why CH4 is non polar. So you can say that ch4 is nonpolar but h2o is polar molecules. The structure of Methane is tetrahedral.
Ch4 is not very much dissolved in water.
 |
| Is Methane Ch4 Polar Or Non Polar Lewis Structure Youtube |
 |
| Solved Ch4 Covalent Compounds Homework Saved 22 The Chegg Com |
 |
| Solved Liilivisuu Iutilii Scilic Question 2 6 2 The Chegg Com |
 |
| Is Ch4 Methane Polar Or Nonpolar Youtube |
 |
| Specify Whether Ch4 Is Polar Nonpolar Ionic Or Polar Covalent Homework Study Com |
Posting Komentar untuk "is ch4 polar"